 Biotech penny stocks have always attracted those who invest in penny stocks due to their explosive nature upon FDA approval of any drugs they have in their pipeline. It’s worth putting them on to a penny stock list to watch, especially when they are not OTC stocks. One of those penny stocks to watch is Durect Corp. (NASDAQ: DRRX). DRRX had a huge drop in share price after announcing results from its Phase 3 trial of POSIDUR in January and has remained relatively stable since.
Biotech penny stocks have always attracted those who invest in penny stocks due to their explosive nature upon FDA approval of any drugs they have in their pipeline. It’s worth putting them on to a penny stock list to watch, especially when they are not OTC stocks. One of those penny stocks to watch is Durect Corp. (NASDAQ: DRRX). DRRX had a huge drop in share price after announcing results from its Phase 3 trial of POSIDUR in January and has remained relatively stable since.
![]() Subscribe today and get our next stock alert delivered to your inbox FREE.
Subscribe today and get our next stock alert delivered to your inbox FREE.
REMOXY is DRRX‘s fastest way to making shareholders rich, or at least richer. Chronic pain affects as many as 50 million Americans annually. Even though pharmaceutical giant Pfizer returned the DRRX drug ELADUR back to the company and the worldwide rights to ELADUR earlier in March, they still are helping to push DRRX’s REMOXY and some analysts on Wall Street think there is value in the equity and its worth taking a longer look at.
OxyContin, accounted for over $3.0 billion in worldwide sales in 2010. REMOXY is designed to replace OxyContin by delivering the same therapy in a form which makes alterations to the drug for abuse and non-prescription use impossible. Wall Street analysts ThinkEquity initiated coverage on NASDAQ listed penny stock DRRX on Friday, March 23. ThinkEquity started their coverage of DRRX with a “buy” rating and $3 price target based on a “sum of all parts” calculation.
DRRX has had a rough time getting their New Drug Application (NDA) submission approved yet the company has a convincing combination of pharmaceutical giant collaborations and an undervalued pipeline that could give shareholders a huge upside if any of them were to get approval. REMOXY could solve a number of problems which have stemmed from abuse of prescription analgesic opioids (PAO) like Oxycontin.
DRRX Stock Snap Shot:
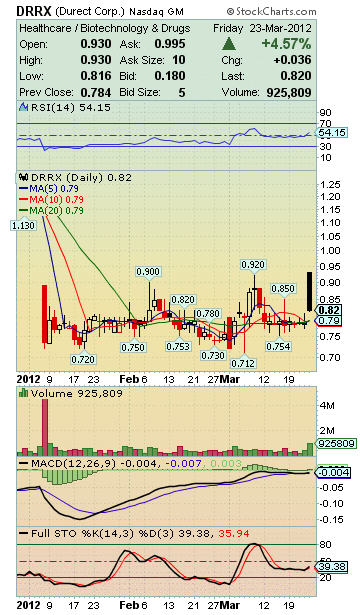 Market Cap: 71.79M
Market Cap: 71.79M
Enterprise Value: 43.36M
Price/Sales (ttm): 2.05
Price/Book (mrq): 19.61
Total Cash (mrq): 28.43M
Total Cash Per Share (mrq): 0.33
52-Week Change: -76.84%
52-Week High (May 2, 2011): 3.77
52-Week Low (Mar 6, 2012): 0.71
50-Day Moving Average: 0.79
200-Day Moving Average: 1.19
Avg Vol (3 month): 451,207
Avg Vol (10 day): 374,812
Shares Outstanding: 87.55M
Float: 69.32M
% Held by Insiders: 17.76%
As of Feb 29, 2012:
Shares Short: 2.45M
Short Ratio: 7.00
Short % of Float: 3.00%
DRRX Stock Price History:
IPO: September 28, 2000
Year ended December 31, 2010:
Q1 – Low: $1.91 High: $3.09
Q2 – Low: $2.23 High: $3.14
Q3 – Low: $2.00 High: $2.58
Q4 – Low: $2.48 High: $3.69
Year ended December 31, 2011:
Q1 – Low: $2.90 High: $3.65
Q2 – Low: $1.76 High: $3.77
Q3 – Low: $1.32 High: $2.28
Q4 – Low: $1.12 High: $1.90
DRRX is a specialty pharmaceutical company which is focused on the development of pharmaceutical products based on the DRRX proprietary drug delivery technology platforms. According to the 10K filed, the DRRX product pipeline currently consists of seven (7) investigational drug candidates that are in clinical development, with one (1) program, REMOXY, the subject of an NDA with the FDA. DRRX received a Complete Response Letter in June 2011 on the REMOXY NDA.
Currently DRRX has one program in Phase III, two programs in Phase II and three programs in Phase I. The potential money makers for DRRX, the more advanced programs, are in the field of pain management. DRRX has other programs underway in fields outside of pain management which include several efforts DRRX has underway which seek to improve the administration of biotechnology agents such as proteins and peptides.
DRRX’s top product and highest in the company’s pipeline is REMOXY. REMOXY is a less-abusable Oxycontin which could possibly be re-filed with the U.S. Food and Drug Administration (FDA) towards the end of 2013. DRRX could file an NDA for the post-surgical pain injection POSIDUR which targets 10 million to 20 million surgeries each year in the second half of 2012. DRRX has even more pain compounds in their deep pipeline which are currently in Phase II or Phase I clinical trials which could also be worthy candidates of NDA submissions.
Regardless, according to the ThinkEquity analyst’s estimates on DRRX; REMOXY alone has been valued at $1.50 a share and Posidur at $1.00 a share. Both values are based on a 5X multiple of estimated 2017 royalties discounted back 5 years at 25% and 40% respectively to account for the development risk to the program. The value of DRRX’s base business, estimated cash at the end of 2012 and the technology value the company holds at $0.50 a share. All values totaled: $3.00.
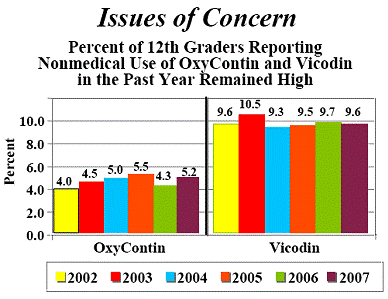
2007 Data but OxyContin Abuse Is Still A Problem
REMOXY (Oral controlled release oxycodone)is a PAO as is POSIDUR (Controlled release injection of bupivacaine). PAO’s are a Multi-Billion Dollar industry in the U.S. alone. Millions of Americans benefit immensely from the prescribed use of PAO’s while more than 10% of Americans have taken PAO’s in an abusive manner at least once in their lives. REMOXY is a pill-shaped, long-acting PAO that has a taffy-like texture which prevents the drug from being physically altered and abused.
Pfizer returned another DRRX drug, ELADUR, and the worldwide rights to it back to DRRX in the beginning of March, 2012. ELADUR is currently in Phase II trials. Pfizer still continues to push and pay royalties to DRRX on REMOXY which it basically inherited via its purchase of King Pharmaceuticals a year ago. REMOXY was developed by Pain Therapeutics (PTIE) and DRRX and both stand to benefit financially from its success through royalty payments.
DRRX’s REMOXY and POSIDUR could reduce PAO abuse due to their design which makes them unalterable. This challenge is one that drug-making giants, including Pfizer, are taking on. The design DRRX has of its pain-killer drugs will ultimately be what makes it a money-maker for both the company and shareholders.
Bottom Line: Durect, Corp., DRRX, is worth adding to the penny stock list of stocks to watch. REMOXY has the backing of Pfizer and could see re-submission of its NDA in late 2013. POSIDUR could see its NDA submission in late 2012. Either way, you know the score: if a drug gets approved, you get paid; if it doesn’t, worst case is you could lose money. At the current trend where DRRX has been trading since mid-January, it seems to have established a bottom so it shouldn’t have too much further down it could go.
ShareMAR

