 “Honey honey, how you thrill me, ah-hah, honey honey” has been the theme for investors who bought into A.P. Pharma Inc. (APPA) prior to January 1, 2012. Without the issuance of one “SOS” all year, APPA stock has steadily climbed from 0.23 making the JMP Securities “outperform” rating and $3.00 price target issued in June look like a “Nina, Pretty Ballerina”. With their “Mamma Mia” $53.55 million dollar insider financing last month, is it too late to say “I Do, I Do, I Do, I Do, I Do?”
“Honey honey, how you thrill me, ah-hah, honey honey” has been the theme for investors who bought into A.P. Pharma Inc. (APPA) prior to January 1, 2012. Without the issuance of one “SOS” all year, APPA stock has steadily climbed from 0.23 making the JMP Securities “outperform” rating and $3.00 price target issued in June look like a “Nina, Pretty Ballerina”. With their “Mamma Mia” $53.55 million dollar insider financing last month, is it too late to say “I Do, I Do, I Do, I Do, I Do?”
![]() Our New Alert Is Set For Mid-September! Click HERE To Make Sure You Don’t Miss It!
Our New Alert Is Set For Mid-September! Click HERE To Make Sure You Don’t Miss It!
 APPA Stock Chart
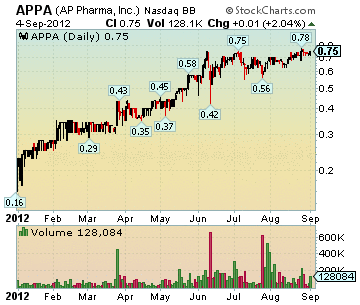
APPA Stock Chart
Market Cap: 226.65M
Close: 0.75, up 0.015 (2.04%)
Volume: 128,084
Dollar Volume: $92,887
High: 0.75
Low: 0.72
Trades: 36
Average Trade Size: 3,557
Authorized: 1,500,000,000
Issued and Outstanding: 302,205,555
![]() Looking for Hot Penny Stocks?
Looking for Hot Penny Stocks?
Click here and get Alerts FREE
Hope the ABBA tunes don’t get stuck in your head from the AP Pharma intro. What hopefully does get stuck in your head is getting APPA on your list of penny stocks to watch immediately.
In July, the Company was allowed 3 new patents by the U.S. Patent and Trademark Office (USPTO) covering the Company’s lead product candidate, APF530. AP Pharma owns the worldwide rights to APF530 and are in the early stages of building the commercial infrastructure necessary to commercialize APF530 in the U.S. on their own. Hence the new financing deal by insiders.
APF530 is being developed for the prevention of both acute- and delayed-onset chemotherapy-induced nausea and vomiting, CINV, one of the most debilitating side effects of cancer chemotherapy and the #1 reason most patients prematurely discontine treatment. To date, there is only one injectable 5-HT3 antagonist approved for the prevention of delayed-onset CINV, so this indication represents an area of particular unmet medical need.
APF530 contains the 5-HT3 antagonist granisetron formulated in the Company’s proprietary Biochronomer™ drug delivery system, which allows therapeutic drug levels to be maintained for 5 days with a single subcutaneous injection. This 5 day range is designed to cover the delayed phase of CINV, whereas the presently available intravenous and oral formulations of granisetron are approved only for the prevention of acute-onset CINV.
In May 2009, Ap Pharma filed a New Drug Application, NDA, with the FDA seeking approval for APF530. The FDA issued a Complete Response Letter for APF530 in March 2010. Since then, the Company has been working to address the issues raised by the FDA and met with the FDA in February and March 2011 to clarify the work needed to resubmit the NDA. Based on their discussions with the FDA and their assessment of the work remaining, AP Pharma plans to resubmit their NDA for APF530 to the FDA this month.
About APPA Stock
A.P. Pharma, Inc.is a specialty pharmaceutical company developing products using its proprietary Biochronomer™ polymer-based drug delivery platform. This drug delivery platform is designed to improve the therapeutic profile of injectable pharmaceuticals by converting them from products that must be injected once or twice per day to products that need to be injected only once every one or two weeks.
Research Points:
Bottom Line: Biotech penny stocks have the potential to generate enomous gains when documentation gets pushed to the FDA. Upon approval, they can go ballistic or conversely implode upon denial. That said, APPA could become a seriously hot penny stock this month and worth every moment of monitoring during the course of this month when they plan to resubmit their NDA.
Here is your opportunity to subscribe to the Most Read Penny Stock Newsletter
If You Can’t Make Money With Us, You Shouldn’t Be Trading Penny Stocks
(We are 100% Anti-Spam and will never rent or sell your information)
ShareSEP

