 One penny stock profiled here as a good penny stock to watch was Generex Biotechnology Corp. (OTCBB: GNBT.OB) who filed their 10-Q yesterday for the quarter ended January 31, 2012. As most biotech companies researching cures to certain cancers, GNBT has been struggling to meet its cash requirements for years but may be on the cusp of announcing a major breakthrough in the fight against breast cancer.
One penny stock profiled here as a good penny stock to watch was Generex Biotechnology Corp. (OTCBB: GNBT.OB) who filed their 10-Q yesterday for the quarter ended January 31, 2012. As most biotech companies researching cures to certain cancers, GNBT has been struggling to meet its cash requirements for years but may be on the cusp of announcing a major breakthrough in the fight against breast cancer.
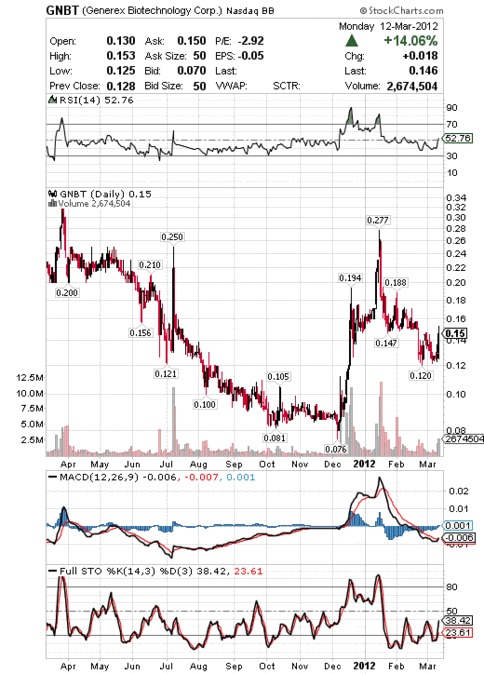
On January 3rd, GNBT reported a regulatory required update on its Phase IIb AE37 breast cancer vaccine study. Our initial alert on GNBT was published January 5 and the stock went from 0.1575 to 0.277, a 75% gain.
GNBT One Year Stock Chart:
GNBT subsidiary, Antigen Express, claimed the interim Phase II data was impressive with a 46% reduction in low HER2 expressing tumors. The report was coupled with a good safety profile to justify ending the Phase II meeting with the FDA which would give Generex an opportunity to move onto the Phase III program. Join Today to Receive Our Next Stock Alert FREE.
 GNBT Stock Snap Shot:
GNBT Stock Snap Shot:
Market Cap: 46.09M
Price/Sales (ttm): 361.19
GNBT Stock Price History:
52-Week High (Mar 29, 2011): 0.33
52-Week Low (Dec 8, 2011): 0.08
5-Day Moving Average: 0.13
10-Day Moving Average: 0.13
20-Day Moving Average: 0.14
50-Day Moving Average: 0.15
200-Day Moving Average: 0.12
GNBT Share Statistics:
Avg Vol (3 month): 2,245,550
Avg Vol (10 day): 1,105,750
Shares Outstanding: 315.71M
Float: 309.94M
% Held by Insiders: 1.47%
GNBT‘s AE37 is an “off-the-shelf” or allogeneic drug which means that it doesn’t have to be customized for each patient which makes it less expensive than competitor Provenge’s $93K price tag for its patient-specific approach (See 10-Q filed here).
AE37 is manufactured from a peptide fragment of the human epidermal growth factor receptor 2 (HER2) protein which is present in roughly 75% of breast cancers. GNBT has not spoken with the FDA about ending the Phase II trial and is not sure to receive the coveted “Special Protocol Assessment” (SPA) designation for its Phase III trial when/if it is allowed.
The SPA designation would define the trial’s endpoints that, if met, would be sufficient for regulatory approval for the drug. These guidelines are critical and will help lead the company through the expensive and time-consuming Phase III regulatory path toward approval.
Bottom Line: GNBT is expected to request permission from the FDA to terminate the Phase II trial sometime before the end of Q1, 2012. When GNBT begins Phase III trial, and the possible SPA designation, makes the difference as to when GNBT impacts shareholder value in the penny stock.
ShareMAR

