 One biotech penny stock to watch that made significant strides last year to position their company as a leading global developer of ophthalmic therapeutics for front-of-eye conditions is OTCBB listed InSite Vision, Inc. (OTCBB: INSV.OB). Their strides have caught the attention of one of Wall Street’s leading analysts, Roth Capital, who yesterday issued a “buy” rating on the biotech stock. All of INSV’s 2012 promises to be another busy and successful year as the hot penny stock gears up its clinical and regulatory efforts focusing toward the goal of facilitating multiple New Drug Applications in 2013.
One biotech penny stock to watch that made significant strides last year to position their company as a leading global developer of ophthalmic therapeutics for front-of-eye conditions is OTCBB listed InSite Vision, Inc. (OTCBB: INSV.OB). Their strides have caught the attention of one of Wall Street’s leading analysts, Roth Capital, who yesterday issued a “buy” rating on the biotech stock. All of INSV’s 2012 promises to be another busy and successful year as the hot penny stock gears up its clinical and regulatory efforts focusing toward the goal of facilitating multiple New Drug Applications in 2013.
INSV initiated the SPA-approved Phase 3 DOUBle clinical study of AzaSite Plus and DexaSite for the treatment of blepharitis, BromSite is poised to begin Phase 3 testing for post-surgical ocular inflammation and the biotech penny stock also advanced pipeline candidates for dry-eye disease in 2011. Their financial results for 2011 proved that when they reported revenues for the year ended December 31, 2011 were $15.9 million compared to $11.9 million for the same period in 2010. Revenues for the fourth quarter of 2011 were $3.1 million compared to $3.9 million for the same period in 2010.
As of December 31, 2011, INSV had cash, cash equivalents and short-term investments totaled $26.4 million. INSV is currently trading at $0.41 on the OTCBB.
InSite Vision Stock Snap Shot:
Market Cap: 54.08M
Prices/Sales: 3.40
52-Week High (Apr 11, 2011): 1.02
52-Week Low (Nov 16, 2011): 0.38
5-Day Moving Average: 0.42
10-Day Moving Average: 0.43
20-Day Moving Average: 0.44
50-Day Moving Average: 0.45
200-Day Moving Average: 0.47
Avg Vol (3 month): 102,934
Avg Vol (10 day): 129,525
Shares Outstanding: 131.91M
Float: 74.69M
% Held by Insiders: 23.11%
Total Cash (mrq): 26.40M
Total Cash Per Share (mrq): 0.20
The company’s AzaSite launched in the U.S. in 2007 by INSV’s commercial partner for the U.S. and Canada; Inspire Pharmaceuticals. INSV’s product development portfolio also includes ISV-502 (AzaSite Plus), which is currently in Phase 3 pivotal trials for the treatment of eyelid infection and inflammation, and additional product candidates leveraging the company’s core DuraSite technology platform.
INSV has recently advanced two new product candidates for the treatment of eye pain and inflammation into preclinical testing.
INSV Two Year Stock Chart:
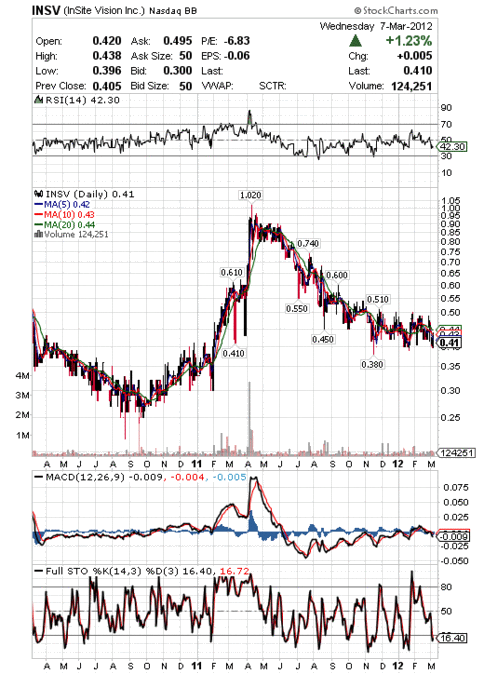
INSV Highlights from 2011:
- In November 2011, INSV initiated the Dual Ophthalmic agents Used in Blepharitis (DOUBle) Phase 3 pivotal trial to evaluate AzaSite Plus and DexaSite simultaneously for the treatment of blepharitis, a common and chronic inflammation of the eyelid. The DOUBle study will seek to enroll approximately 900 patients suffering from moderate-to-severe blepharitis in a four-arm trial designed to evaluate the efficacy and safety of both product candidates. INSV obtained a Special Protocol Assessment from the U.S. Food and Drug Administration in May 2011. To date, INSV has enrolled 211 patients in the DOUBle study and expects to complete the trial and announce top-line results in late 2012 or early 2013.
- In November 2011, INSV announced that a U.S. Patent and Trademark Office (USPTO) panel of judges found in favor of the company in its patent interference litigation with the University of California, San Francisco (UCSF), and have confirmed the inventorship of INSV‘s U.S. Patent Nos. 6,239,113 and 6,569,443 protecting AzaSite. In January 2012, UCSF filed an appeal of the USPTO’s judgment in favor of INSV.
- In October 2011, INSV announced positive top-line results from a Phase 2 pharmacokinetic study comparing the tissue penetration profile of INSV‘s BromSite (ISV-303; 0.075% bromfenac in DuraSite) with ISTA Pharmaceuticals’ Bromday (bromfenac ophthalmic solution) 0.09%. Study results showed that BromSite administered once-daily achieves greater than twice the tissue penetration of Bromday. INSV plans to initiate a Phase 3 clinical trial of BromSite in mid-2012.
- AzaSite (azithromycin ophthalmic solution) 1% royalties for the fourth quarter of 2011 were $2.2 million compared to $3.3 million in same period of 2010. AzaSite is marketed in the U.S. by Merck for the treatment of bacterial conjunctivitis. The AzaSite royalty under Merck decreased by 32 percent during the fourth quarter of 2011 compared to the same period in 2010. For the full year, AzaSite royalties totaled $13.9 million in 2011 compared to $10.7 million in 2010, representing a decrease of six percent in net sales of AzaSite which was offset by the $3.9 million minimum royalty true-up payment from Merck.
- Besivance (besifloxacin ophthalmic suspension) 0.6%, marketed by Bausch + Lomb for the treatment of bacterial conjunctivitis, saw significant increases in both prescription and revenue amounts compared to the fourth quarter 2010. INSV recorded approximately $0.5 million of Besivance royalty revenue in the fourth quarter of 2011 compared to $0.1 million in same period of 2010. Year to date, Besivance royalties were $1.2 million in 2011 compared to $0.5 million in the same period of 2010.
Bottom Line: INSV‘s strategy includes maximizing AzaSite sales in the U.S. and abroad through close collaboration with marketing partners, seeking development partners for its DuraSite-enabled early-stage product candidates, and applying the company’s expertise in ophthalmology to identify, in-license, or acquire promising new product candidates or technologies.
Add INSV to your stock watch list immediately and make sure to conduct your own due diligence on this OTCBB listed biotech penny stock and approach it as a long-term hold more so than a one-day flip.
ShareMAR

