 While everyone trading penny stocks is trying to find the next great penny stock, savvy investors looking for OTC stocks will be watching closely Talon Therapeutics, Inc. (OTCBB: TLON.OB). Talon Therapeutics, Inc. is a S.F. Bay Area based biopharmaceutical company. TLON, formerly known as Hana Biosciences, has experienced an expected run over the last few months leading up to the AdComm panel decision. TLON is going to be one of the penny stocks to watch today as it awaits word from the FDA on Marqibo, the company’s leukemia drug.
While everyone trading penny stocks is trying to find the next great penny stock, savvy investors looking for OTC stocks will be watching closely Talon Therapeutics, Inc. (OTCBB: TLON.OB). Talon Therapeutics, Inc. is a S.F. Bay Area based biopharmaceutical company. TLON, formerly known as Hana Biosciences, has experienced an expected run over the last few months leading up to the AdComm panel decision. TLON is going to be one of the penny stocks to watch today as it awaits word from the FDA on Marqibo, the company’s leukemia drug.
TLON has an incredibly low float and has a tremendous run in 2012 but, as always, betting on biotech penny stocks with new drugs coming up for FDA approval has long been a game played by those investing in penny stocks. The decision is scheduled to be announced to Talon Therapeutics Wednesday for Marqibo, its drug candidate for the treatment of adult Philadelphia chromosome-negative acute lymphoblastic leukemia.
![]() Subscribe today and get our next stock alert delivered to your inbox FREE.
Subscribe today and get our next stock alert delivered to your inbox FREE.
As with all penny stocks on the rise, TLON has some interesting points that raise both eyebrows and the hair on the back of your neck at the same time. TLON stock closed up 10.23% on Friday at 0.97. TLON is up over 100% this year. And with a float of less than 20 million shares, TLON is set-up for what could be (Baseball Analogy) either a home run or hit-by-pitch broken wrist which requires surgery and causes you to be out for the next 3-6 months on the stock.
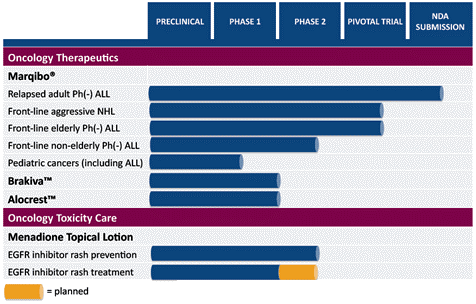
The FDA only approved 21 new drugs in 2010, 35 last year and 2012 has investors lined up to take advantage of all the companies like TLON that have Phase II and Phase III trials in progress. Over the last decade, 2011 was the second highest number of new drugs the FDA approved in a single year. TLON stands strong on its leukemia drug, Marqibo, which the company hopes to receive a favorable answer from the FDA by Wednesday. TLON also has three other drugs that are in Phase II trials.
TLON Drug Pipeline:
See the TLON Schedule and applications here:
Calendar
UCM296398.pdf
UCM296399.pdf
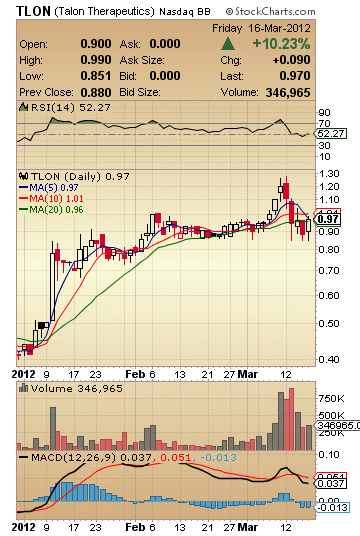 TLON Stock Snap Shot:
TLON Stock Snap Shot:
Market Cap: 21.12M
52-Week High (Apr 29, 2011): 1.65
52-Week Low (Dec 30, 2011): 0.40
5-Day Moving Average: 0.97
10-Day Moving Average: 1.01
20-Day Moving Average: 0.96
50-Day Moving Average: 0.93
200-Day Moving Average: 0.71
Avg Vol (3 month): 160,570
Avg Vol (10 day): 534,625
Shares Outstanding: 21.78M
Float: 19.29M
% Held by Insiders: 21.00%
What raise the eyebrows after reading and seeing the stock chart of TLON is that Marquibo is to be used to treat non-Hodgkin’s lymphoma and other forms of acute lymphoblastic leukemia (A.L.L.) including pediatric cancers. TLON is currently performing clinical trials for:
- Brakiva to treat small cell lung and ovarian cancers;
- Alocrest to treat lung and breast cancers; and
- Menadione topical lotion for rash prevention due to skin toxicity from cancer treatments.
What raises the hair on the back of one’s neck is that the number of patients with A.L.L. is not huge. In the U.S., there are roughly 4,000 new cases each year. Furthermore, the 30-day mortality in the Marqibo study was 12% which may be cause for concern. 32% of the treated patients reported serious febrile neutropenia, a fever brought on by infections caused by abnormally low white blood cells. 22% suffered from serious neuropathy, or nerve damage/pain.
In 2010, TLON executives presented their data to the American Society of Clinical Oncology regarding Marqibo. At the time, the 20% complete response rate outweighed historical single-digit response rates observed in similar, advanced A.L.L. patients that were also treated with current single-agent therapies. FDA granted approval of Clolar which was manufactured by Genzyme based on a similar 20% complete response rate. The difference though is Clolar was approved for pediatric A.L.L. while Marqibo is hoping to get approval for patients over 18.
Two other interesting findings are that:
- TLON has requested a Special Meeting of Stockholders of Talon Therapeutics, Inc.to be held on April 5, 2012. The meeting is to gain approval from the shareholders to increase the number of authorized shares of common stock from 350,000,000 to 600,000,000. The request is in order to be able to convert previously sold preferred shares to Warburg Pincus Purchasers and Deerfield Purchasers.
- James E. Flynn, on behalf of the Deerfield Purchasers Group, sold the following blocks of TLON since the beginning of 2012:
Mar. 14th: 35,526 at $1.01 per share (Proceeds of $35,881)
Mar. 13th: 18,500 at $1.04 per share (Proceeds of $19,240)
Mar. 12th: 123,180 at $1.11 per share (Proceeds of $136,729)
Mar. 9th: 153,000 at $1.20 per share (Proceeds of $183,600)
Mar. 8th: 55,000 at $1.04 per share (Proceeds of $57,200)
Mar. 7th: 21,000 at $0.99 per share (Proceeds of $20,790)
Feb. 6th: 25,000 at $0.98 per share (Proceeds of $24,500)
There are a long list securities sold by Mr. Flynn dating as far back as May, 2011 and a substantial number of shares still held by the Deerfield Purchasers which makes the above seem reasonable. However, with pending response from the AdComm panel decision and hopeful FDA approval of Marqibo, would a possible investor look to this as cause for concern or business as usual?
You can research TLON here
Bottom Line: TLON is a must add to your stock watch list today, Monday, March 19. The FDA will release the briefing documents on Marqibo to the Adcomm panel which will include the FDA’s review of the Marqibo data. Expect TLON to be one of the more volatile stocks traded based on the findings of the FDA review.
Wednesday the actual FDA panel is scheduled from 8am to 12:45 pm EDT. Expect TLON to be halted for trading Wednesday until after the panel ends. Pending the outcome, this could be a home run shot with TLON or a strike out but one thing is for certain, TLON will not be 0.97 come Wednesday afternoon.
ShareMAR

